Electronic Data Capture System Rave
An electronic data capture EDC system also referred to as electronic case report form eCRF is a very important software tool in clinical trials. Real-time electronic data capture EDC to ensure data quality and compliance across all sites.
Https Www Medidata Com Wp Content Uploads 2019 05 Customer Facing Fact Sheet Rave Edc May 19 1 Pdf
One of the team members got an interesting thought to seek this feedback quickly and much more reliably.
Electronic data capture system rave. Simplicity combined with complex customizable workflows. Is to create a Partnership with Sponsors to establish electronic data capture system using Medidata RAVE and clinical data management process that are easy to use and implement. We can establish your company URL in 1 week and have your study designed depending on complexity in 2 weeks with all the necessary edit checks.
The top-rated Electronic Data Capture system Maximize productivity by easily capturing processing and integrating data from multiple sources on a compliant electronic data capture EDC system. False Summary for Electronic Data Capture System InForm GTM. Rave EDCはMedidata Rave Clinical Cloudの基盤であり短時間での実装によりあらゆる規模期間複雑な試験であってもサポートが可能ですRave EDCは17000件以上の試験現在進行中の3分の1と400万人以上の被験者の実績によりそれが証明されています 当社の柔軟なアーキテクチャはデータの取得からクレンジング管理までを臨床研究チームが一気通貫でできる.
This gives some idea of what the market considers are this systems competitors. Or A computerized system design for the collection of data in electronic format for use mainly in human. EDC Electronic data capture EDC.
Clinical research relies on accurate data and EDC solutions are used to collect clean and analyze the data produced in clinical studies. Automatic set-up of eCRFs and data validation plan by uploading CDISC metadata. Somewhere in the western world a clinical trial team of an organization was looking to buy an electronic data capture EDC system for their clinical trial projects.
Dynamic lab ranges with support for central and. Flexible for any type of study easy protocol amendment management Reduces set up time. REDCap RAVE CRFs should only capture data that is specified in the approved protocol Every data point collected on a CRF requires complementary source data in order to be verified 9.
It may refer to mobile applications used by financial institutions to collect digital signatures. There were many choices in the market and efforts were on to seek feedback on previous experien6ces with these products. These groups include in addition to ECOG-ACRIN the Alliance for Clinical Trials in Oncology Childrens Oncology Group NRG Oncology and SWOG.
The process of collecting data into a permanent electronic form. Permanent in the context of these definitions implies that any changes made to the electronic data are recorded via an audit trail. CRFs can be paper or electronic eg.
Rave Electronic Data Capture EDC System Take advantage of the industrys leading data engine. See also data entry data acquisition. Castor is the top-rated Electronic Data Capture EDC system.
What is Electronic Data Capture Software. Electronic data capture software allows field teams surveyors researchers and others to collect and submit data via a mobile handheld device. The Data Managers build the specification and build the EDC database.
Edcsystem clinicalresearch electronicdatacapture clinicalgyanEDC- Electronic Data Capture Systems as mainstream in clinical researchThis video explains. Version 100 User. Castors EDC eConsent ePRO eCOA Digital Enrollment and eSource solutions enable researchers to easily capture and integrate data from any source and decentralize their trials.
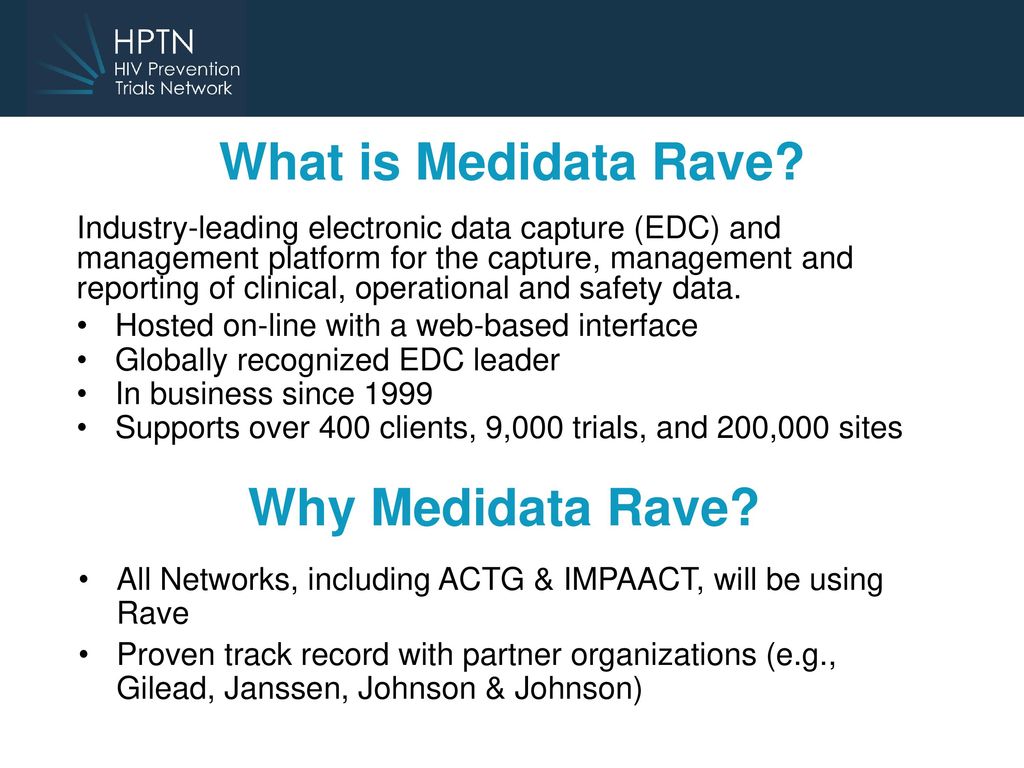
Introducing Medidata Rave Karen Patterson M. Rave is the standard electronic data capture system for all network groups in the NCI National Clinical Trials Network.
Electronic Data Capture Introducing Medidata Rave Karen Patterson

Top 5 Alternatives To Medidata Rave August 2021 Saasworthy Com

Medidata Rave User Manual Data Capture System Medidata Rave Summary For Electronic Data Capture System Medidata Rave
Http Www Iacct2017 Com Sites 386 Editor Documents Liora 20bosch Pdf

Medidata Rave Edc Ctms Integration Simpletrials Clinical Trial Management System

Electronic Data Capture Introducing Medidata Rave Ppt Video Online Download

Electronic Data Capture Introducing Medidata Rave Ppt Download

Edc Electronic Data Capture Systems Youtube

Using Edc Rave To Conduct Clinical Trials At Genentech Ppt Video Online Download
Electronic Data Capture Introducing Medidata Rave Karen Patterson

Edc System For Clinical Trials Cdisc Compliant Iddi
Electronic Data Capture Introducing Medidata Rave Ppt Download
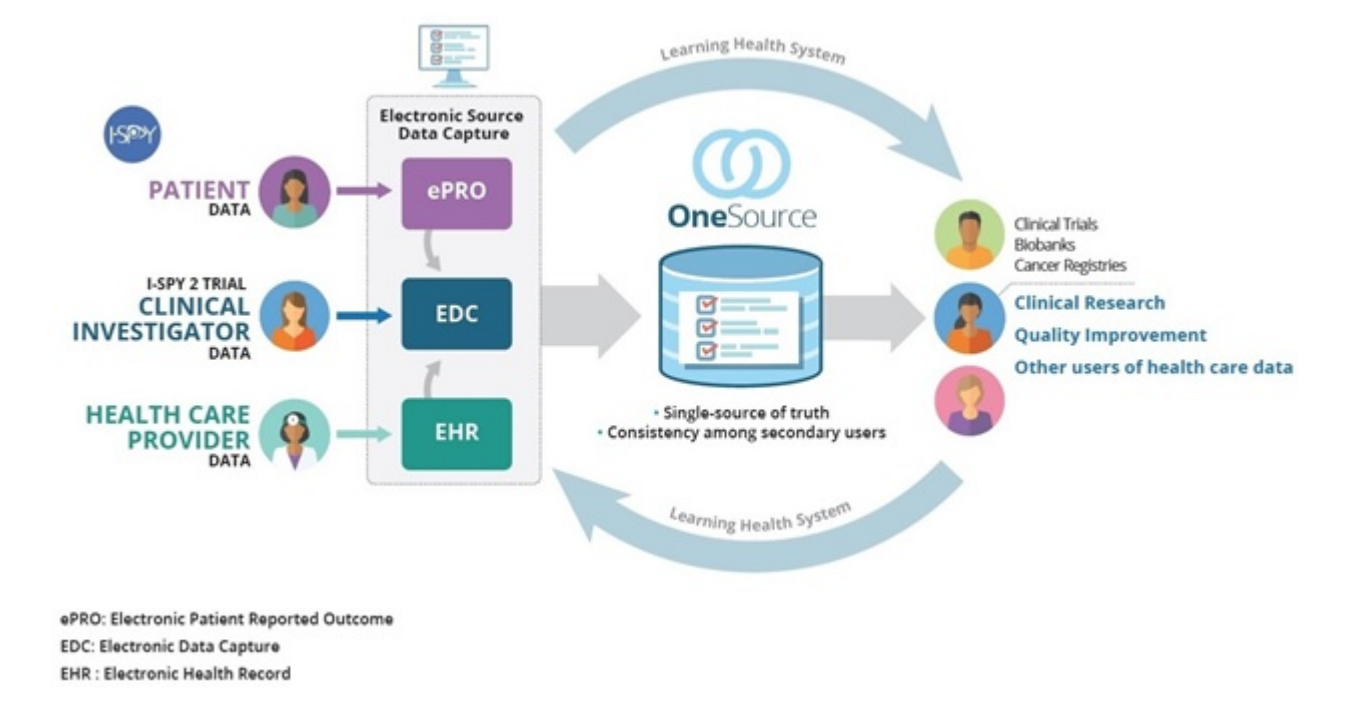
Clinical Trial Software Edc Ctms Epro Rtsm Altexsoft
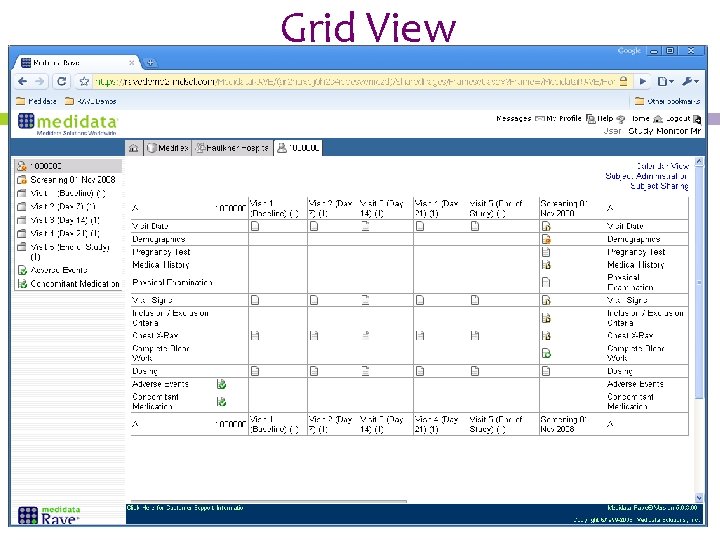
Electronic Data Capture Introducing Medidata Rave Karen Patterson
Https Www Medidata Com Wp Content Uploads 2018 12 Fact Sheet Rave Edceasyfast Flexible Powerful Cdm Pdf

Electronic Data Capture Introducing Medidata Rave Karen Patterson
Https Www Medidata Com Wp Content Uploads 2019 05 Customer Facing Fact Sheet Rave Edc May 19 1 Pdf
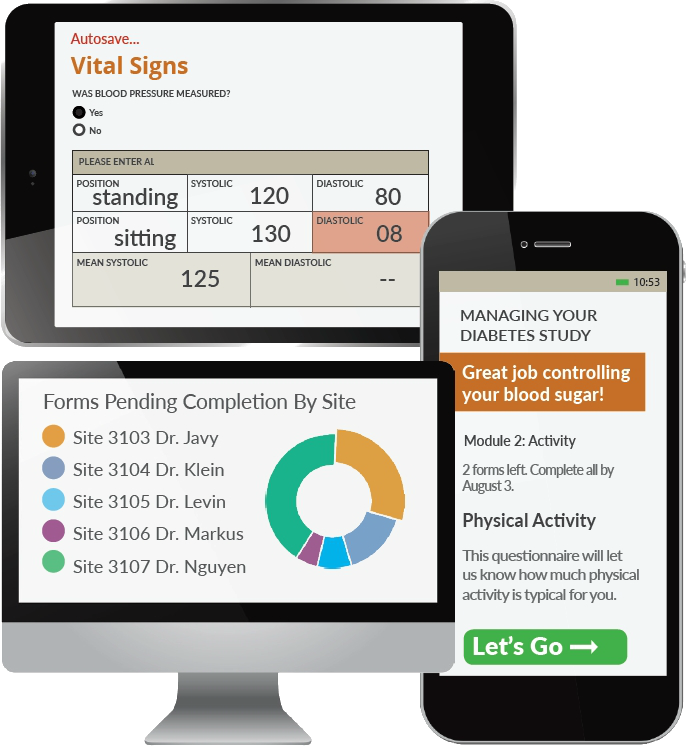
Electronic Data Capture The Fundamentals Of Edc Openclinica

Using Edc Rave To Conduct Clinical Trials At Genentech Ppt Video Online Download
Post a Comment for "Electronic Data Capture System Rave"