Electronic Data Capture (edc) Definition
At OpenClinica we are immersed in EDC all day every day. Key features include WYSIWYG form editor risk-based monitoring activity d.
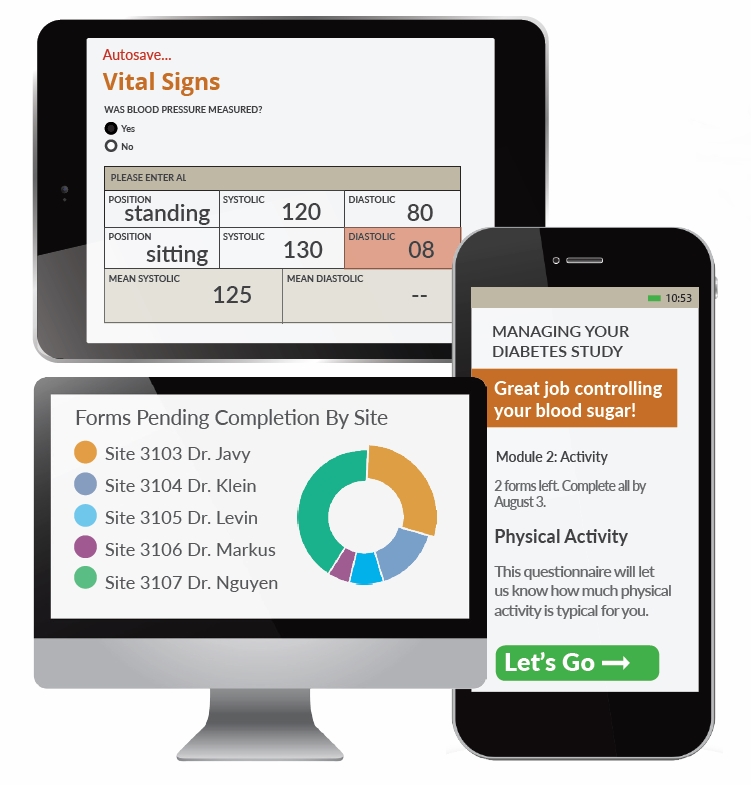
Electronic Data Capture The Fundamentals Of Edc Openclinica
Electronic data capture EDC is the computerized collection and management of clinical trial data from patients and subjects.

Electronic data capture (edc) definition. They are used to gather sufficient patient data during the testing of new pharmaceuticals. Electronic Data Capture EDC Electronic data capture EDC is the electronic acquisition of clinical study data using data collection systems such as Web-based applications interactive voice response systems and clinical laboratory interfaces. Electronic Data Capture for clinical investigations and post market clinical follow-up.
La aiie électronique de donnée EDC et la collecte et la getion informatiée de donnée deai clinique de patient et de ujet. Now they are replaced by Electronic Data Capture systems. They sound the same.
A reporting tool for analysis of the collected data. To put it simply an Electronic Data Capture EDC system is software that stores patient data collected in clinical trials. Electronic Data Capture EDC is the medical abbreviation is admittedly a fairly generic sounding term but in the clinical trials field it actually means something fairly specific.
Annonce Electronic Data Capture thats tailor-made for MedTech studies. Big Data pour les grandes et petites entreprises. An Electronic Data Capture EDC system is a computerized system designedfor the collection of clinical data in electronic format for use mainly in human clinical trials.
Direct data capture DDC and electronic data capture EDC. Using systems to collect clinical trial data in electronic form as opposed to paper form. During investigational site audits special systems for data entry provided by the pharmaceutical company are evaluated.
EDC systems represent computerized systems that collect data in electronic format. A validation component to check user data. EDC is the current technology used by research institutions sponsors and CROs to manage clinical trial data when using electronic trial data handling andor remote electronic trial data systems.
Electronic Draft Capture EDC In e-commerce efficient technology is critical to successful customer experience. Some of the most fundamental EDC functionalities comprise of quality checking of already collected data allowing. Source Data Capture.
Data is typically first recorded on paper and is then transcribed into the system and saved in an electronic case report form eCRF. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Captivate EDC is a cloud-based electronic data capture solution that assists medical researchers and practitioners with data capture and forms management.
An EDC system uses technology to streamline the collection and transmission of clinical trial data from the patient to the research laboratory. Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. A graphical user interface component for data entry.
What Does Electronic Data Capture EDC Mean. But when you dig a little deeper there are fundamental crucial distinctions that make a significant difference in clinical research workflows data quality and timelines. What is an EDC system.
Electronic Data Capture for clinical investigations and post market clinical follow-up. Level up your career in data capturing structural assessments and lifecycle analysis. As speed of pay and convenience escalate in the industry reliable quick-moving technology is imperative for merchants both in an establishment and online.
Typically EDC systems provide. Many consumers prefer to pay using a card so merchants need the ability to accept this popular method of payment. In Clinical Pharmacology units we see EDC-Systems which support the study conduct and are used for documentation of data.
Annonce Electronic Data Capture thats tailor-made for MedTech studies. Transcription of Data From Paper or Electronic Sources to the eCRF Duplication of data sources Increased likelihood of data errors. Principal La Technologie Capture de données électroniques EDC Capture de données électroniques EDC 2021.
Un ytème EDC utilie une technologie pour rati. EDC systems are used by life sciences. Level up your career in data capturing structural assessments and lifecycle analysis.
An eSource system can be considered as an EDC Electronic Data Capture system. During CRO-Audits eg.
Auditing Electronic Data Capture In Clinical Trials Edc

Electronic Data Capture And The Fight Against Covid 19

Electronic Data Capture Edc Workflow For Data Sharing In Neuroimaging Download Scientific Diagram

Ppt Edc Electronic Data Capture Powerpoint Presentation Free Download Id 6675075
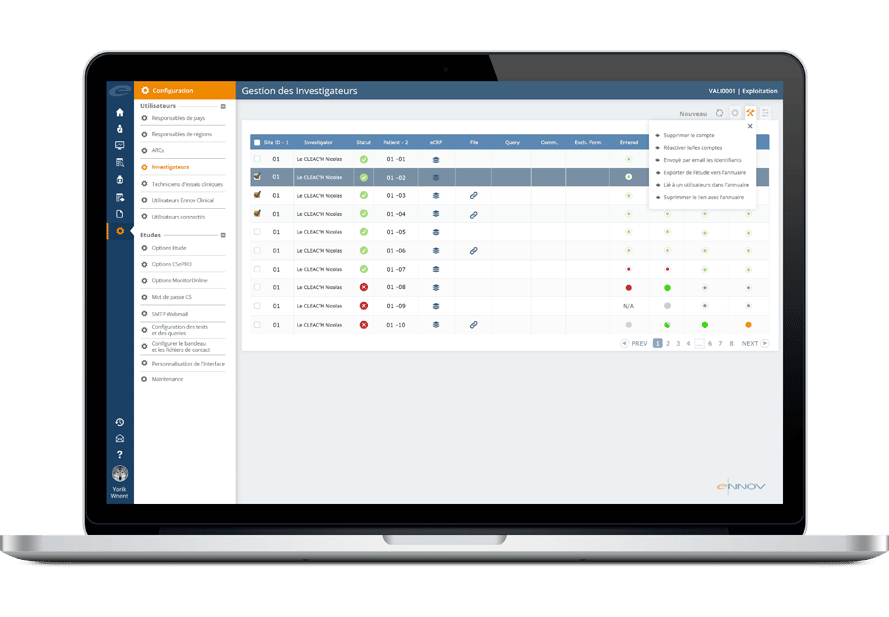
Edc Electronic Data Capture Software Ennov Software For Life
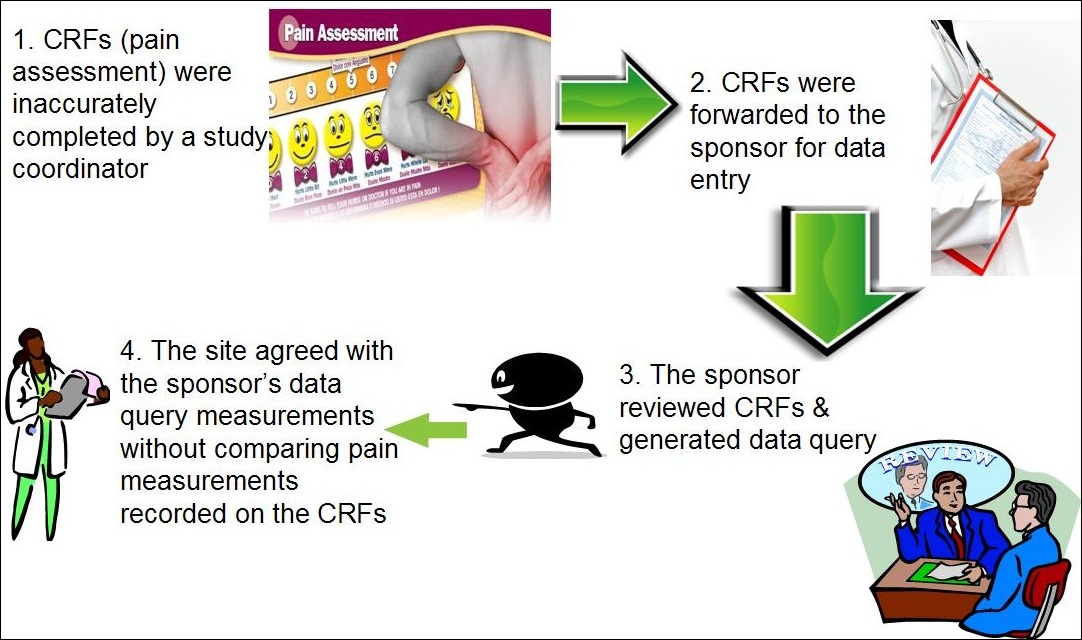
The Future Of Clinical Trials Using Electronic Data Capture Systems Socra

Pdf Electronic Data Capture In Clinical Trials Interface Design And Evaluation And System Validation Semantic Scholar

Electronic Data Capture Edc Software Bioclinica
Pestronk Electronic Data Capture Selecting An Edc System Journal Of The Society For Clinical Data Management
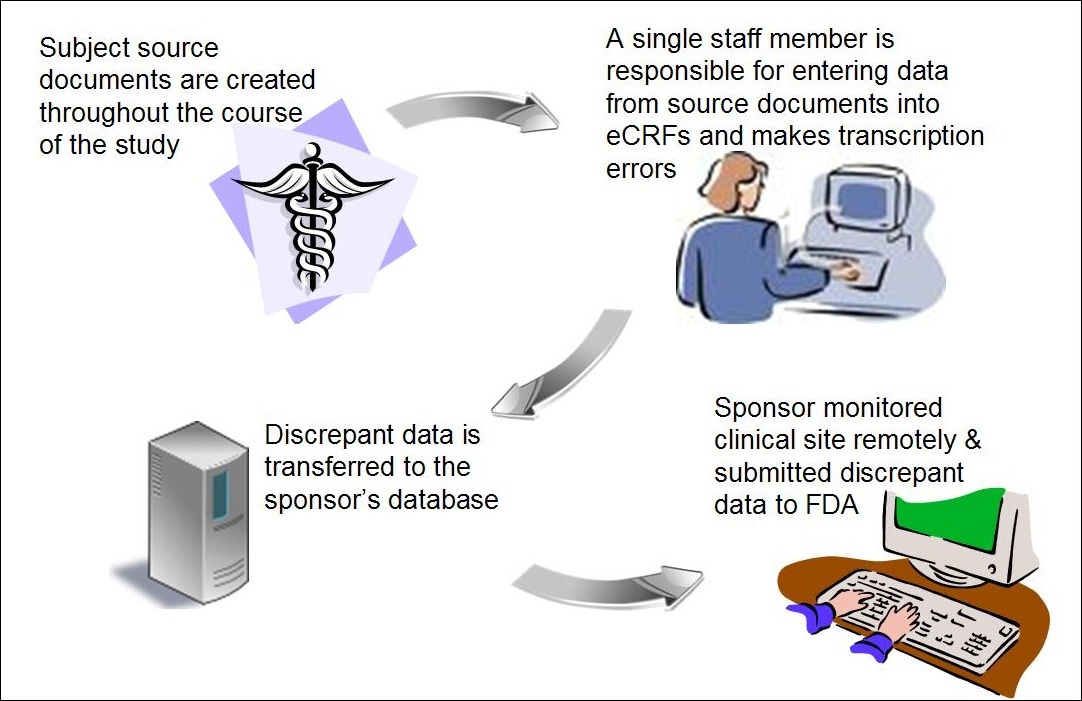
The Future Of Clinical Trials Using Electronic Data Capture Systems Socra

Pdf Electronic Data Capture In Clinical Trials Interface Design And Evaluation And System Validation Semantic Scholar

Auditing Electronic Data Capture In Clinical Trials Edc
Electronic Data Capture Edc Openspecimen
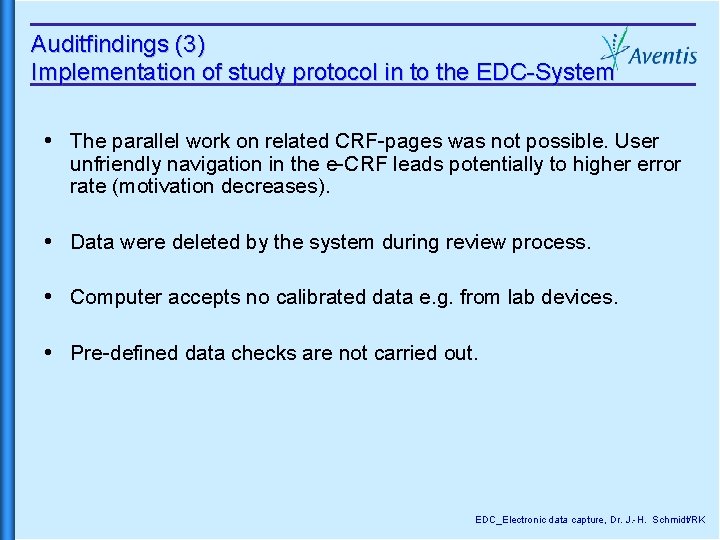
Auditing Electronic Data Capture In Clinical Trials Edc

What Is A Electronic Data Capture Terminal Erp Fm

User Selects An Electronic Data Capture System Edc From The Dropdown Download Scientific Diagram
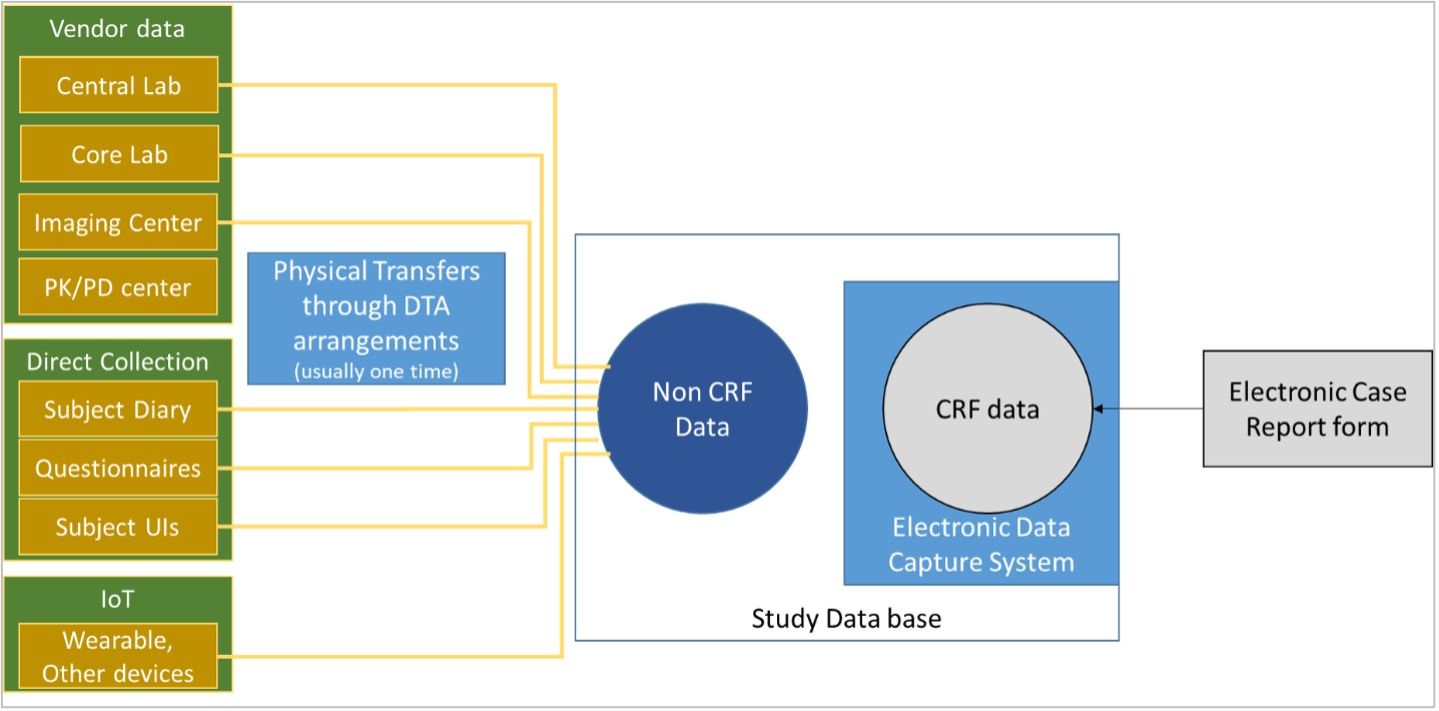
Electronic Data Capture In Clinical Trials
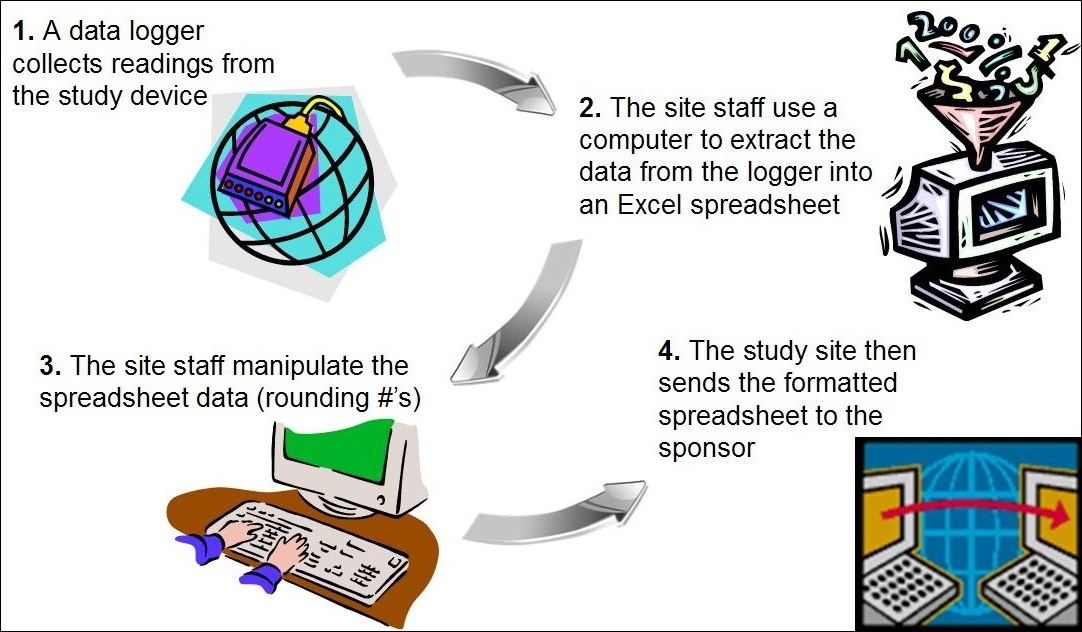
The Future Of Clinical Trials Using Electronic Data Capture Systems Socra
Post a Comment for "Electronic Data Capture (edc) Definition"