Electronic Medication Management System Governance And Standards
Medication Safety require that health service organisations have governance arrangements. Clinicians use the safety and quality systems from the Clinical Governance Standard when.

Download Pdf Essentials Of Health Information Management Principles And Practices By M Health Information Management Book Essentials Electronic Health Records
MM010101-The organization plans its medication processes.

Electronic medication management system governance and standards. Electronic Medication Management Systems A Guide to Safe Implementation 2nd edition ACSQHC Sydney 2012. Many of the risks associated with each part of the medication management pathway can be avoided by using systems and processes. What makes IT Governance Europe a trusted provider When it comes to protecting your information assets youre safe with us.
Implementing policies and procedures for medication management. The Australian Commission on Safety and Quality in Health Care ACSQHC wishes to acknowledge the contribution of participating clinicians and organisations in the development and review of this guide. Governance and risk management 2.
The combination of CareMeds systems with the patented MultiMeds blister deliver this. The Medication Safety Standard requires health service organisations to assess medication management and implement processes and practices that. A guide to safe implementation includes guidance on scoping system selection configuration implementation training and ongoing operation.
Managing risks associated with medication management. We describe the implementation of an electronic medication management system eMMS in an Australian teaching hospital to inform future similar exercises. A guide to safe implementation third edition published by the Commission in 2017.
Technology recommendations self-assessment checklists and action planning tools for the three key areas five case studies from within NHSScotland which illustrate different NHS board approaches and experiences to business planning andor implementing electronic prescribing and. A DTC is a multidisciplinary committee that is committed to the overall governance of the medicines management system to ensure the judicious appropriate safe effective and cost-effective use of medicines in their health service organisation1 Scope These Guiding. Timely system response to user.
CareMeds is the only Person Centered System that gives. Medication Safety Standard 4 Part 2 â Governance and systems for medication safety Margaret Duguid Pharmaceutical Advisor Graham Bedford Medication Safety Program Manager. Annonce Convenient Free Easy To Use List of Best Electronic Mgmt Systems.
The Electronic Medication Management Systems Business Requirements the Requirements are an annexe to the Electronic Medication Management Systems. We are a leading provider of cyber risk and privacy management solutions and have built a strong global presence with our deep technical expertise and proven track record. Hospital medication management is a complex process involving many steps prescribing dose calculation dispensing preparation and administration.
Networks to expand their existing medication management governance processes to incorporate safe and effective electronic medication management eMeds design use and ongoing optimisation. Electronic Medication Management Systems. Provide for sound governance for the safe and quality use of medicines.
The success of eMMS implementation depends on. Identifying training requirements for medication management. They strive to achieve the best possible outcomes for their patients and residents by delivering a person centred medication management solution.
Electronic Medication Management Systems A Guide to Safe Implementation 2nd edition 2012. Means use of the eMeds system is an approved form of prescribing under the Regulation. These Guiding Principles aim to.
A positive workplace culture leadership teamwork and clinician ownership acceptance of the major impact on work practices by all staff. Leadership and organisational change 3. Minimise the occurrence of medicine-related incidents and the potential for patient harm from medicines.
Replaces current individual eMeds system approval by the Ministry for Health for use at specified hospitals. The organization has written policies that describe medication management processes that is accessible to Licensed Independent Practitioners LIPs and staff who are involved in the patients medications. We put the person at the centre of everything we do and share access to the Pharmacy and Care Provider.
NSW Health policy mandates that the DTC1 of a hospital local health district LHD or speciality health network provide local governance of medication-related processes. Electronic Medication Management eMeds Electronic Medication Management eMeds systems support the improved quality safety and effectiveness of medication management with NSW hospitals. This includes providing support for doctors nurses and pharmacists to prescribe order check reconcile dispense and record the administration of medicines.
Health service organisations procure medicines for safety. Compliance with this Electronic Medication Management System Governance and Standards Procedure. Clinicians are supported to supply store compound manufacture prescribe dispense administer monitor and safely dispose of medicines.
Slide 1 Medication Safety Standard 4 Part 2 Governance and systems for medication safety Margaret Duguid Pharmaceutical Advisor Graham Bedford Medication Safety Program. Organisation and Standard 4. The Requirements aim to support hospitals and health service organisations to procure and assess EMM systems.
Https Www Ema Europa Eu En Documents Other Eudravigilance Operational Plan Milestones 2020 2022 En Pdf

Pin On Electronic Health Records

Medisecure In The Ehealth Landscape Medisecure Ehealth Prescription It Service Provider
Https Joinup Ec Europa Eu Sites Default Files Inline Files Digital Public Administration Factsheets Sweden Vfinal Pdf

Identity Access Management Market Insights On Size Share Demand Trends Key Players Cyber Security Software Management Identity

Central Governemnt Job For Msc Graduates Integrative Integrative Medicine Job
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Product Management Services Pms Implementation International Organization Standardization Iso En Pdf
Https Www Myhealthrecord Gov Au Sites Default Files Clinical Governance Framework V2 5 Pdf V 1566786544
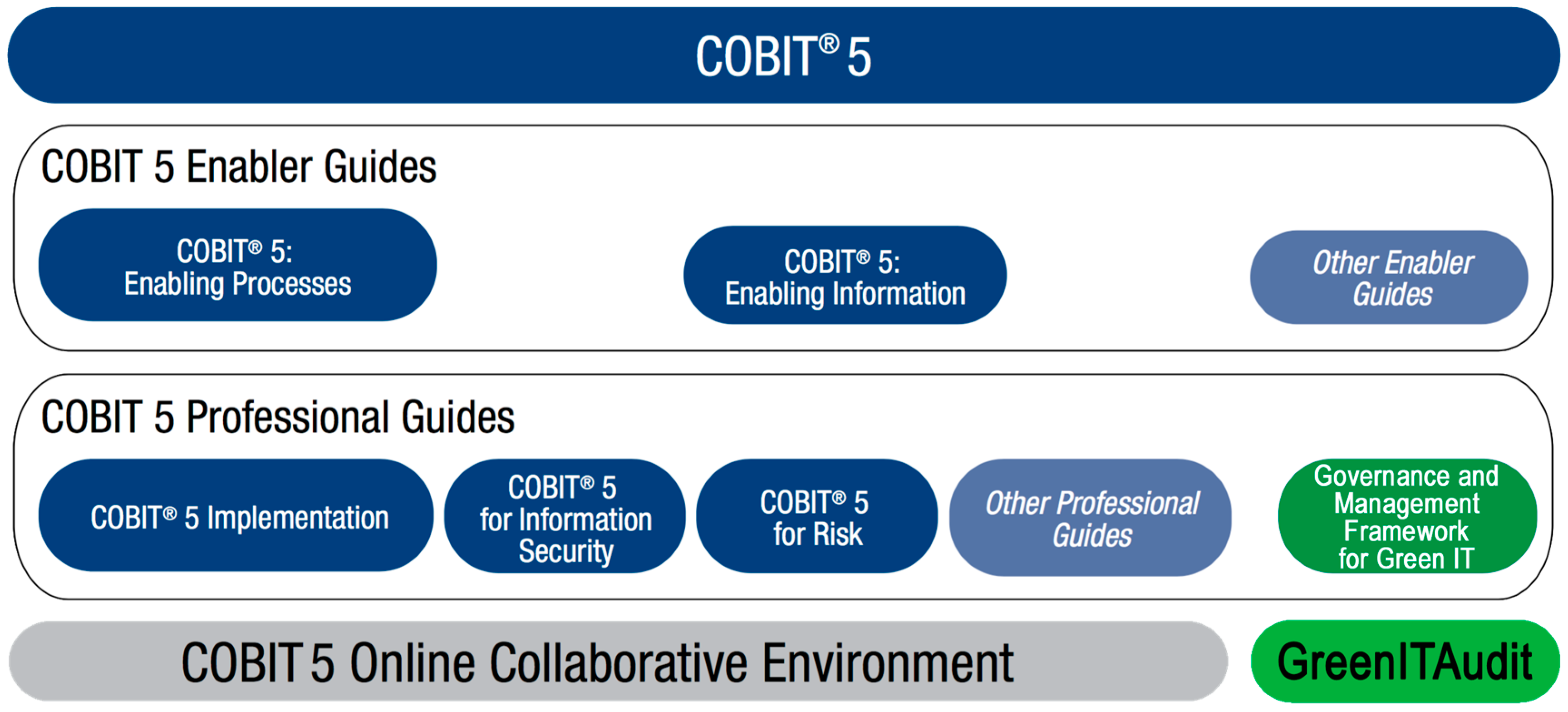
Sustainability Free Full Text A Governance And Management Framework For Green It Html

Figure 1 Life Cycle Of An Electronic Resource Records Management Content Distribution Resource Management

Top Enterprise Data Strategy Company In The Usa Testing Strategies Strategies Enterprise

Risk Management An Overall And Continuing Systematic Process For The Assessment Control Communication And Review O In 2021 Risk Management Risk Analysis Management

Electronic Record An Overview Sciencedirect Topics
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf

Industrial Hygiene Sop Guideline Occupational Health And Safety Hygiene Occupational Health

Document Strategies Building A Framework Strategies Life Cycle Stages Business Content

Ata Quality Streamlining Business Processes Providing Information Access And Making Electronic Health Information Systems Data Quality Business Intelligence

Electronic Record An Overview Sciencedirect Topics

Post a Comment for "Electronic Medication Management System Governance And Standards"