Electronic Data Capture System Definition
A validation component to check user data. Using systems to collect clinical trial data in electronic form as opposed to paper form.
To put it simply an Electronic Data Capture EDC system is software that stores patient data collected in clinical trials.
Electronic data capture system definition. A graphical user interface component for data entry. An Electronic Data Capture EDC system is a computerized system designedfor the collection of clinical data in electronic format for use mainly in human clinical trials. Electronic Data Capture for clinical investigations and post market clinical follow-up.
The process of using computer equipment or software to read and store information from documents. Annonce Electronic Data Capture thats tailor-made for MedTech studies. Annonce Electronic Data Capture thats tailor-made for MedTech studies.
It streamlines data management and helps replace traditional paper-based forms. Electronic data capture the system for scheduling and encountering client information. Learn best-practices and common pitfalls in mission planning of data capturing.
EDC systems are used by life sciences. Electronic Data Capture EDC is the medical abbreviation is admittedly a fairly generic sounding term but in the clinical trials field it actually means something fairly specific. What is an EDC system.
Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Arizona Arthritis Center in Tucson described the Outcomes Research Management Information System. Learn best-practices and common pitfalls in mission planning of data capturing.
Electronic Data Capture for clinical investigations and post market clinical follow-up. An EDC system uses technology to streamline the collection and transmission of clinical trial data from the patient to the research laboratory. Data is typically first recorded on paper and is then transcribed into the system and saved in an electronic case report form eCRF.
Edcsystem clinicalresearch electronicdatacapture clinicalgyanEDC- Electronic Data Capture Systems as mainstream in clinical researchThis video explains. Typically EDC systems provide. A reporting tool for analysis of the collected data.
Electronic data capture définition signification ce quest electronic data capture. De très nombreux exemples de phrases traduites contenant electronic data capture system Dictionnaire français-anglais et moteur de recherche de traductions françaises. EDC software is an electronic data capture solution that lets surveyors clinical researchers and other field teams collect data via electronic forms.
Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Data captured by the tool is stored at a centralized location and can be fed into other software applications for analysis or reporting. Le système recueille des données électroniques pour la planification des activités et pour trouver des renseignements sur lusager.
They are used They are used to gather sufficient patient data during the testing of new pharmaceuticals. EDC systems represent computerized systems that collect data in electronic format. Electronic data capture EDC is the computerized collection and management of clinical trial data from patients and subjects.
Electronic data capture system - Traduction française Linguee.
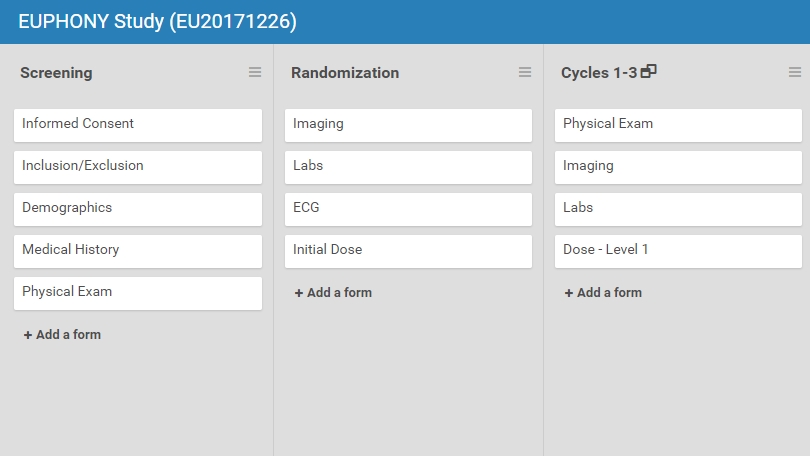
Openclinica Enterprise Edc System Clinical Data Management

What Is Mis Characteristics Objectives Role Component
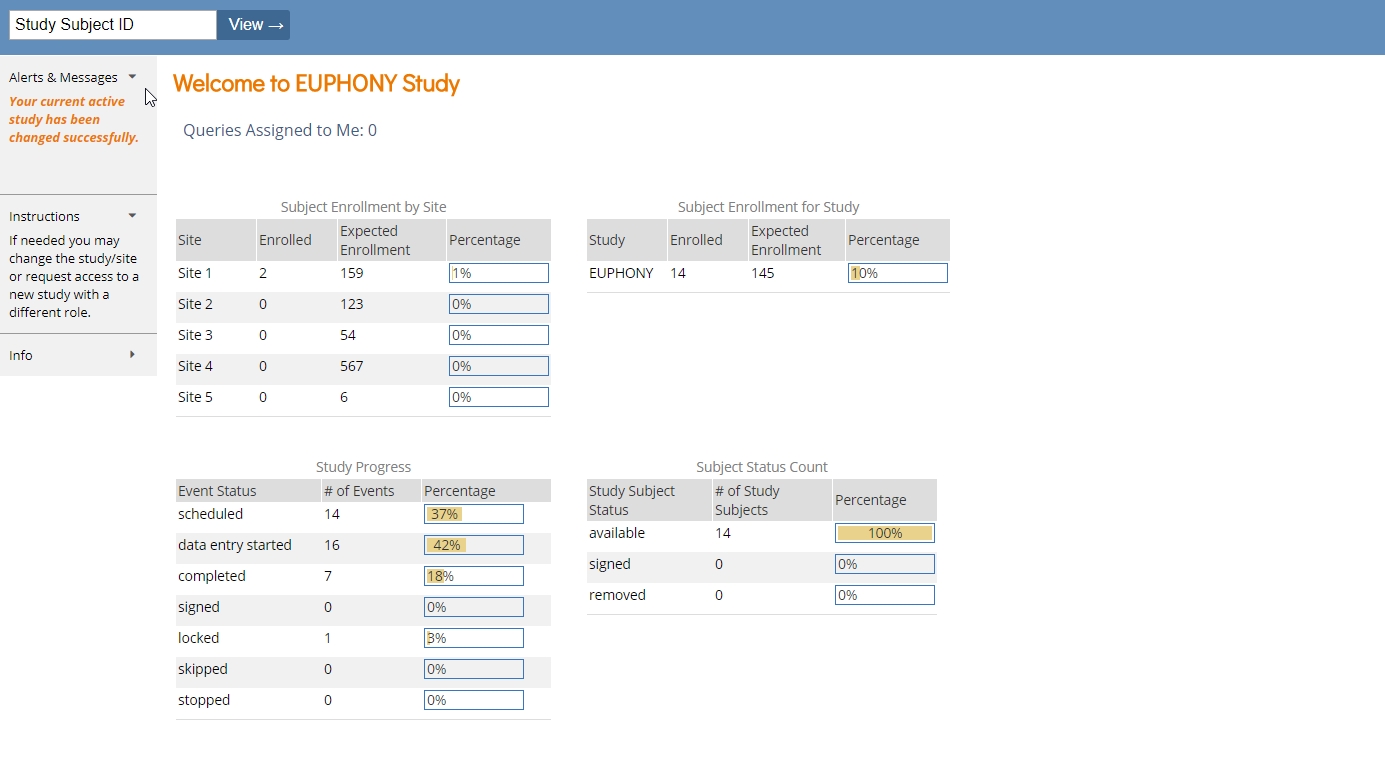
Openclinica Enterprise Edc System Clinical Data Management
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor

What Is Invoice Processing Definition Steps Flowchart Software

Gs1 Global Traceability Standard Gs1
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
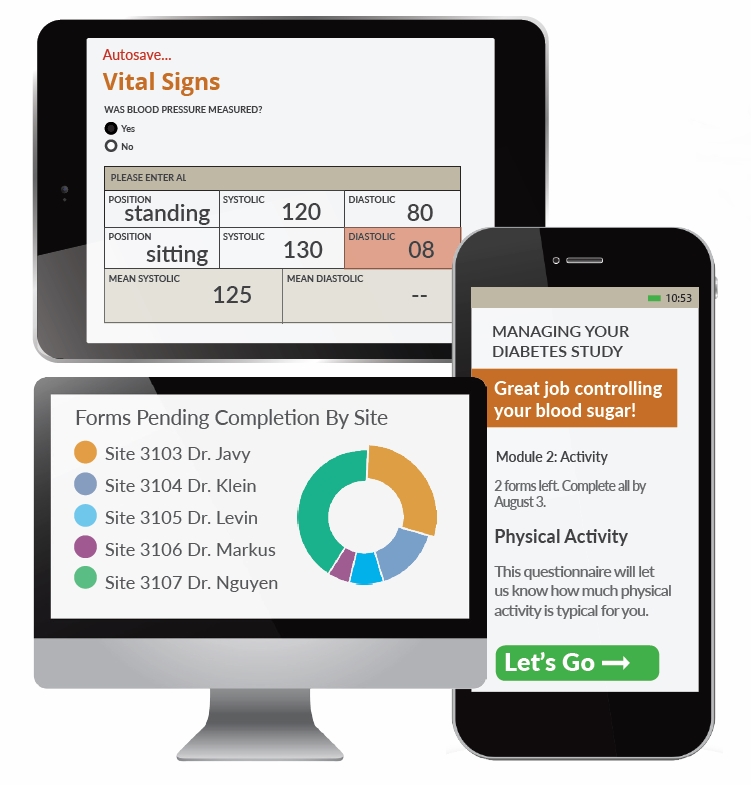
Electronic Data Capture The Fundamentals Of Edc Openclinica

What Is Ecoa And How Does It Improve Clinical Trial Data Quality

What Is Ecoa And How Does It Improve Clinical Trial Data Quality
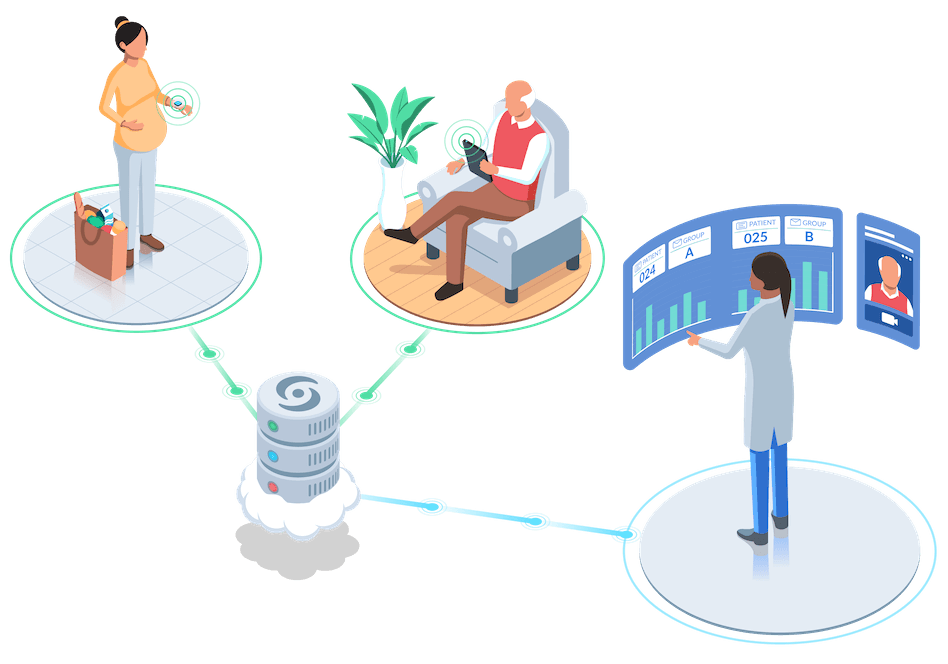
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor

Gs1 System Architecture Document Gs1
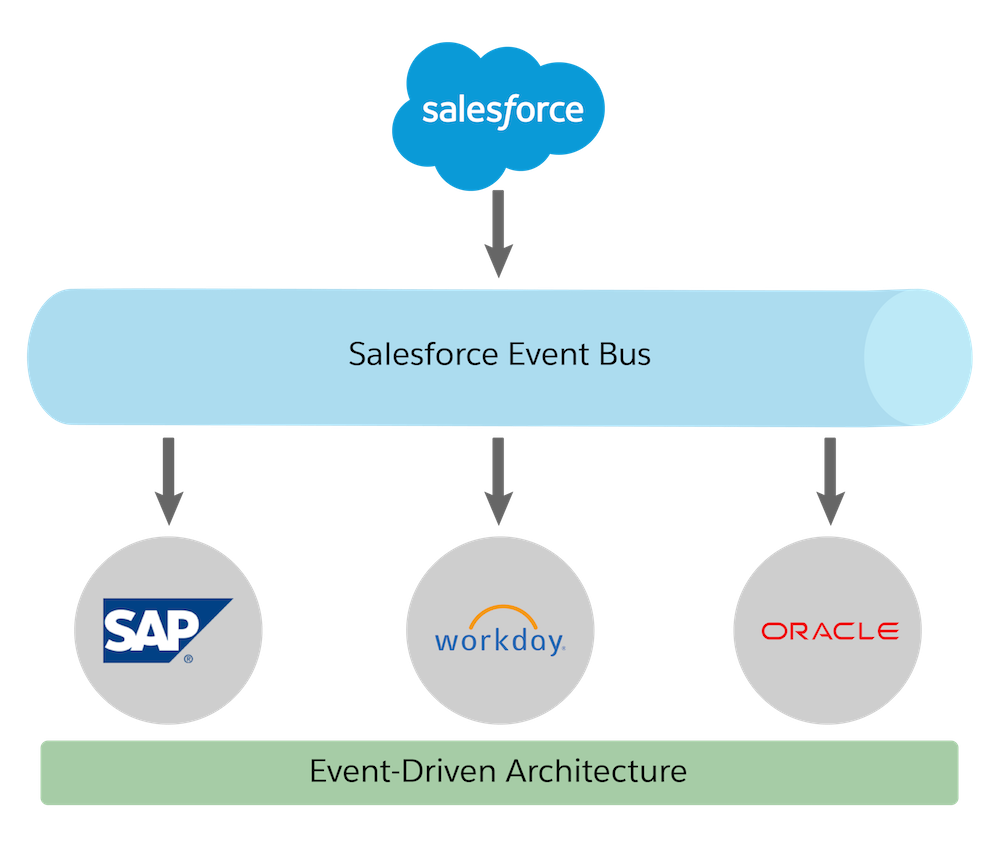
Understand Change Data Capture Unit Salesforce Trailhead

Gs1 System Architecture Document Gs1
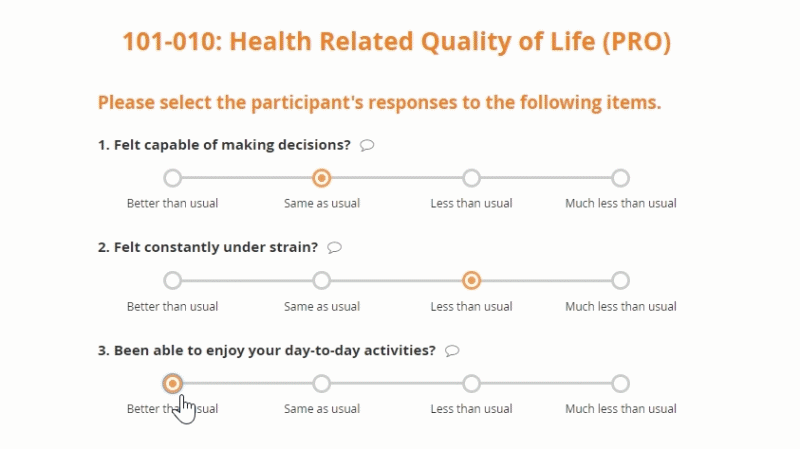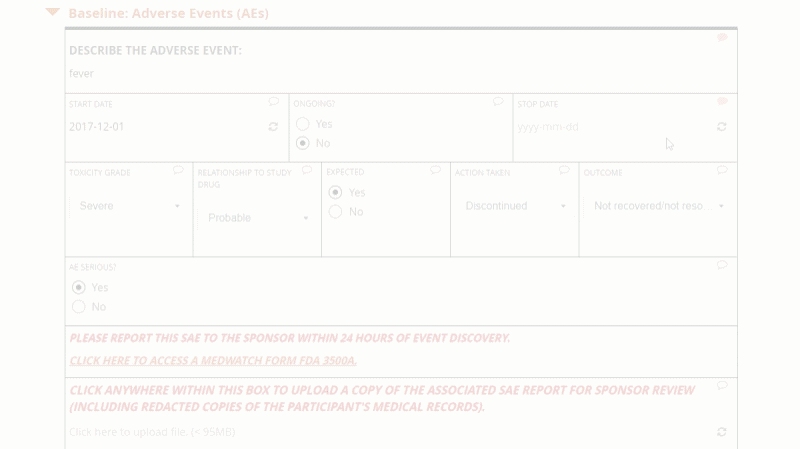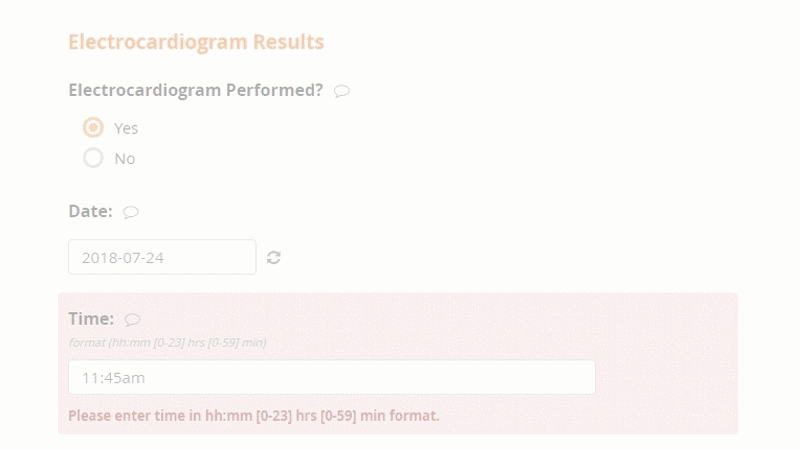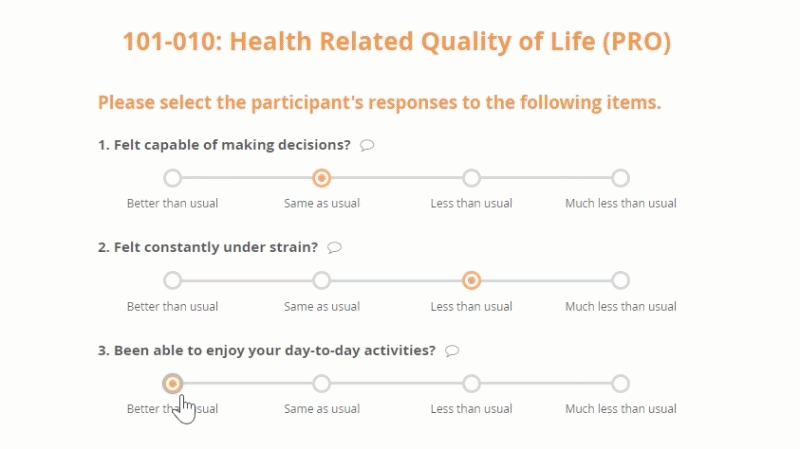
Openclinica Enterprise Edc System Clinical Data Management
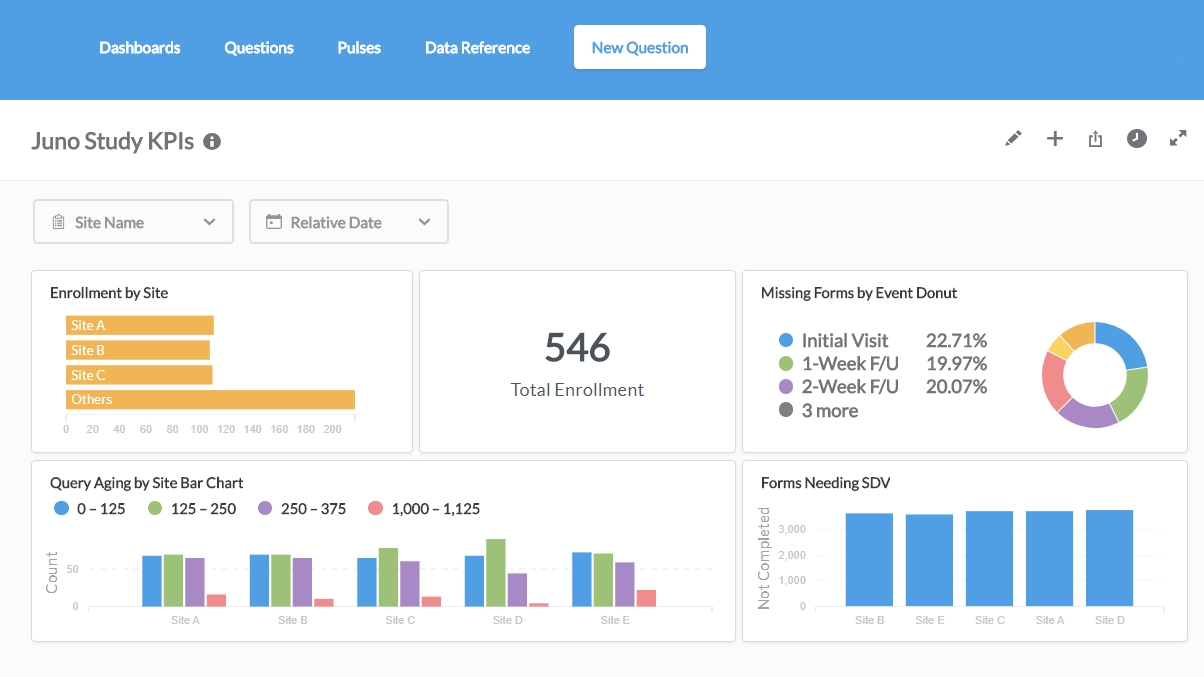
Openclinica Enterprise Edc System Clinical Data Management

Post a Comment for "Electronic Data Capture System Definition"