Electronic Data Capture Systems Examples
Annonce Review a Free List of the Best Electronic Data Capture Products - Start Today. We present an overview of the issues to consider when selecting an electronic data capture system and a survey of available software solutions.

8 Apps For Field Data Collection Teamscope Odk Etc
Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing.
Electronic data capture systems examples. Data is typically first recorded on paper and is then transcribed into the system and saved in an electronic case report form eCRF. EDC systems are typically used for data collection for clinical trials but. We also discuss how to select an electronic data capture system.
Annonce STRUCINSPECT DCE Data Capture Expert training for remote data capturing. Castor is an Electronic Data Capture system for medical research. Annonce Electronic Data Capture for clinical investigations and post market clinical follow-up.
In addition it speeds up the entire clinical trial. Annonce Electronic Data Capture for clinical investigations and post market clinical follow-up. Automated data capture can be conducted via different systems since by definition data occurs in multiple formats eg being encrypted into QR codes.
Electronic Data Capture thats tailor-made for MedTech studies. Annonce Review a Free List of the Best Electronic Data Capture Products - Start Today. Examples of Electronic Data Capture systems Medrido Castor EDC Aetiol DataLabs Poimapper In conclusion the Pharmacy sector is adapting new electronic-oriented practices just like any other industry nowadays.
These tools are called EDC systems. To put it simply an Electronic Data Capture EDC system is software that stores patient data collected in clinical trials. The process reduces data errors to provide researchers with improved data quality.
Electronic Data Capture thats tailor-made for MedTech studies. At OpenClinica we are immersed in EDC all day every day. Examples of Electronic Data Capture systems.
Electronic Data Capture EDC systems are used to collect store and manage data generated from clinical trials or other research studies in electronic forms which can either replace the traditional paper forms or be used in conjunction with paper forms for which data is then transcribed into the electronic forms. It streamlines data management and helps replace traditional paper-based forms. Electronic Data Capture EDC is the medical abbreviation is admittedly a fairly generic sounding term but in the clinical trials field it actually means something fairly specific.
More and more clinical trials are making the move to EDC software and replacing paper records with electronic. Data captured by the tool is stored at a centralized location and can be fed into other software applications for analysis or reporting. Using systems to collect clinical trial data in electronic form as opposed to paper form.
Learn best-practices and common pitfalls in mission planning of data capturing. An EDC system uses technology to streamline the collection and transmission of clinical trial data from the patient to the research laboratory. The number of related use cases in retail and in-store payment is skyrocketing.
Top features include self service eCFR creation form building randomization ePro patient surveys monitoring easy import and export of data EHR data importer. It may refer to mobile applications used by financial institutions to collect digital signatures. It may also refer to applications used by clinicians and researchers to collect observed or subject data during a clinical trial.
Options range from simple stand-alone spreadsheets to full service relational databases controlled by certified database administrators. EDC software is an electronic data capture solution that lets surveyors clinical researchers and other field teams collect data via electronic forms. Trying to deliver better outcomes while utilizing their own work research specialists are altering traditionally accepted methods and choosing computerized versions instead.
However its important to recognize that many participants in the field of clinical trials are. Castors EDC eConsent ePRO eCOA Digital Enrollment and eSource solutions enable researchers to easily capture and integrate data from any source and decentralize their trials. Investigators encounter numerous challenges when initiating a research project including selecting an appropriate electronic data capture system.
Built by researchers for researchers Castor is an easy to use complete and affordable solution that offers all the capabilities researchers expect from an EDC and more. Castor is the top-rated Electronic Data Capture EDC system. In conclusion the Pharmacy sector is adapting new electronic-oriented practices just like any other industry nowadays.
Industry leaders like Walmart Starbucks and Amazon turned the technology into a novel solution for capturing data on merchandises and making. Learn best-practices and common pitfalls in mission planning of data capturing. What is an EDC system.
Trying to deliver better outcomes while utilizing their own work research. Electronic data capture EDC is the computerized collection and management of clinical trial data from patients and subjects. Electronic data capture software allows field teams surveyors researchers and others to collect and submit data via a mobile handheld device.
18 Examples Of Big Data In Healthcare That Can Save People
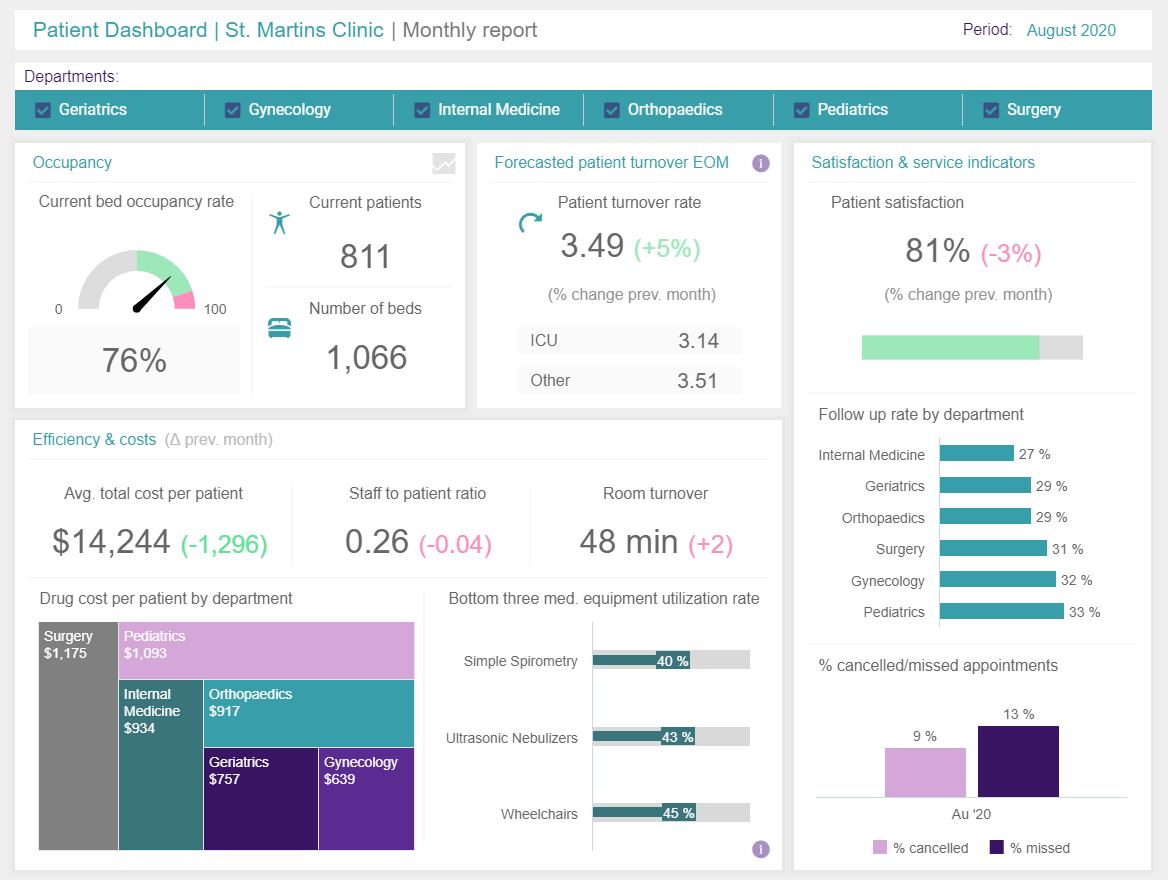
18 Examples Of Big Data In Healthcare That Can Save People
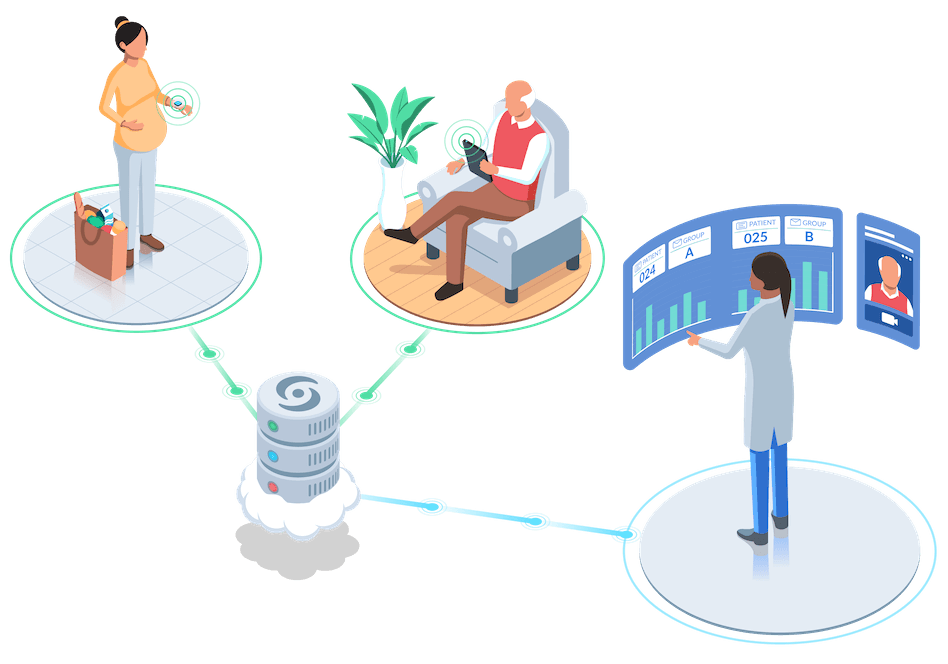
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
View Of Internet Based Data Collection Promises And Realities Journal Of Research Practice
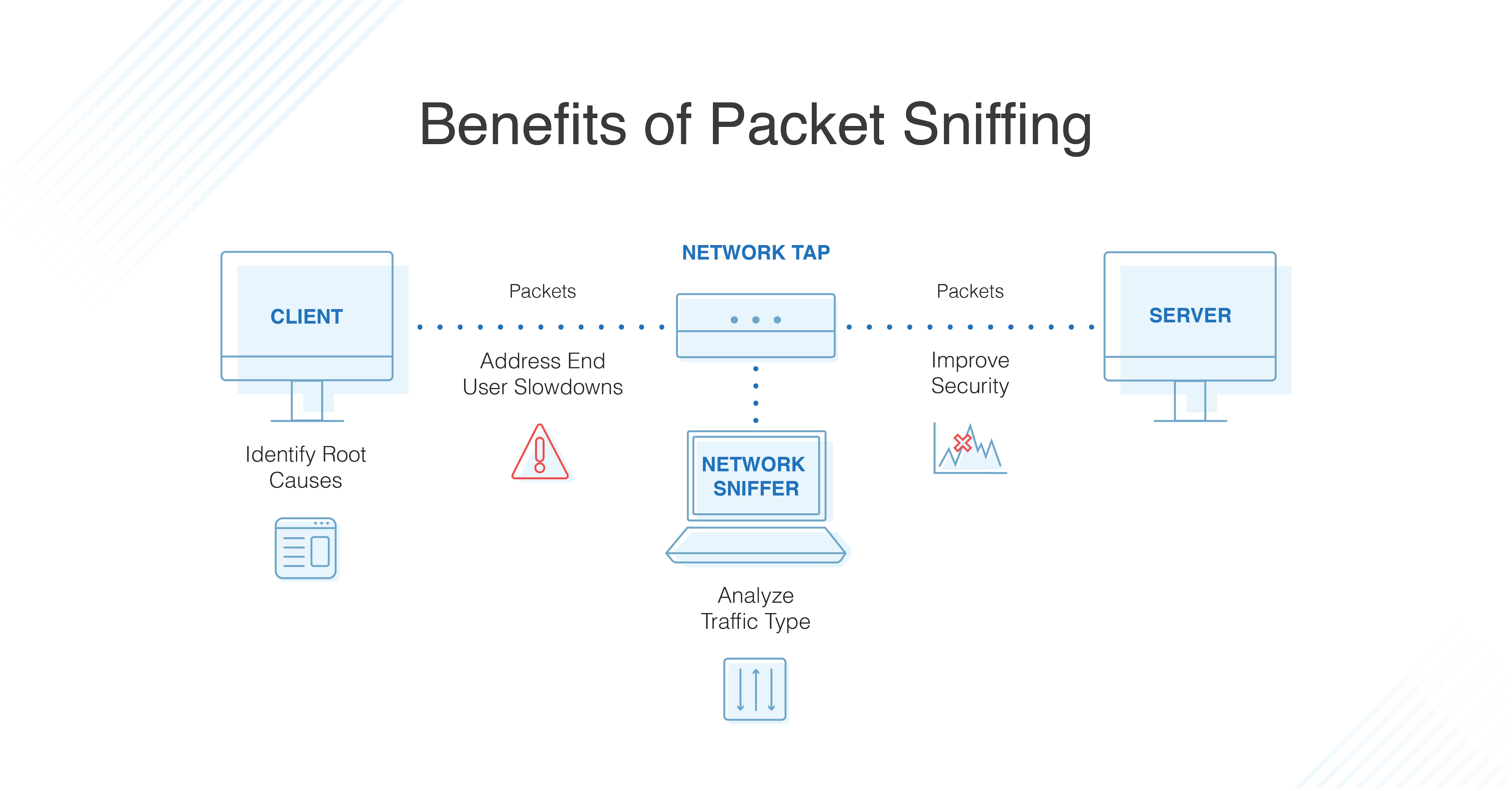
10 Best Packet Sniffers Comparison And Tips Dnsstuff

8 Apps For Field Data Collection Teamscope Odk Etc

Pin On Gds Ware Field Data Capture
Https Www Ema Europa Eu En Documents Regulatory Procedural Guideline Draft Guideline Computerised Systems Electronic Data Clinical Trials En Pdf
Electronic Data Capture Edc Econsent Ecrf Epro Clinical Research Castor

8 Apps For Field Data Collection Teamscope Odk Etc

What Is Ecoa And How Does It Improve Clinical Trial Data Quality

Electronic Data Interchange Learning Electronic Data Systems Data Electronics
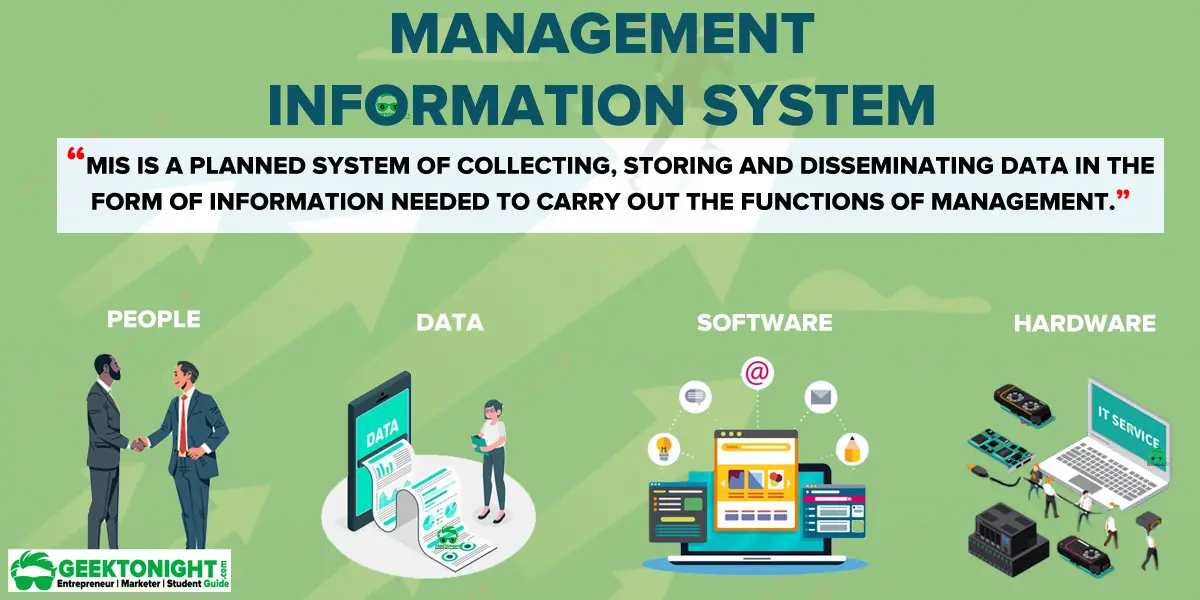
What Is Mis Characteristics Objectives Role Component

Gs1 System Architecture Document Gs1
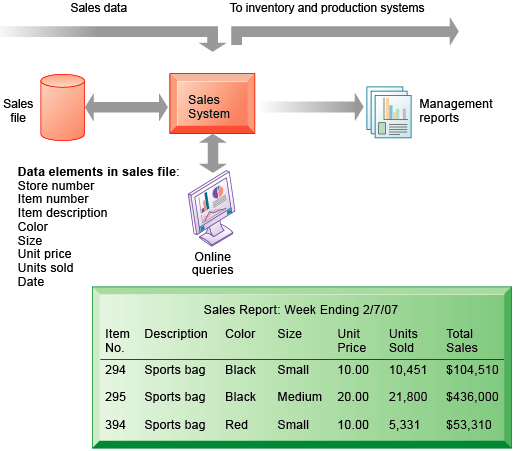
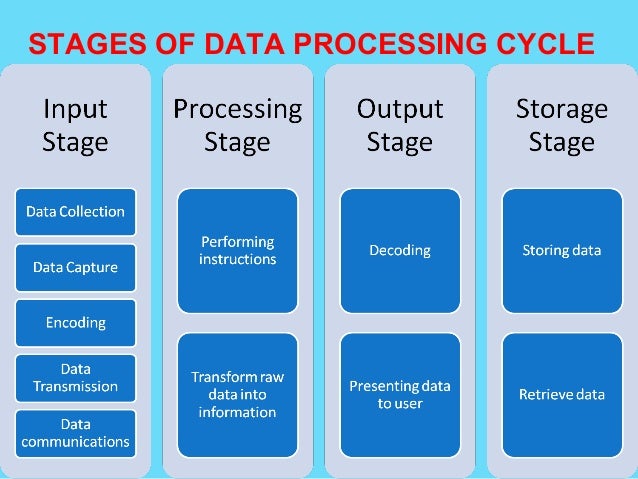
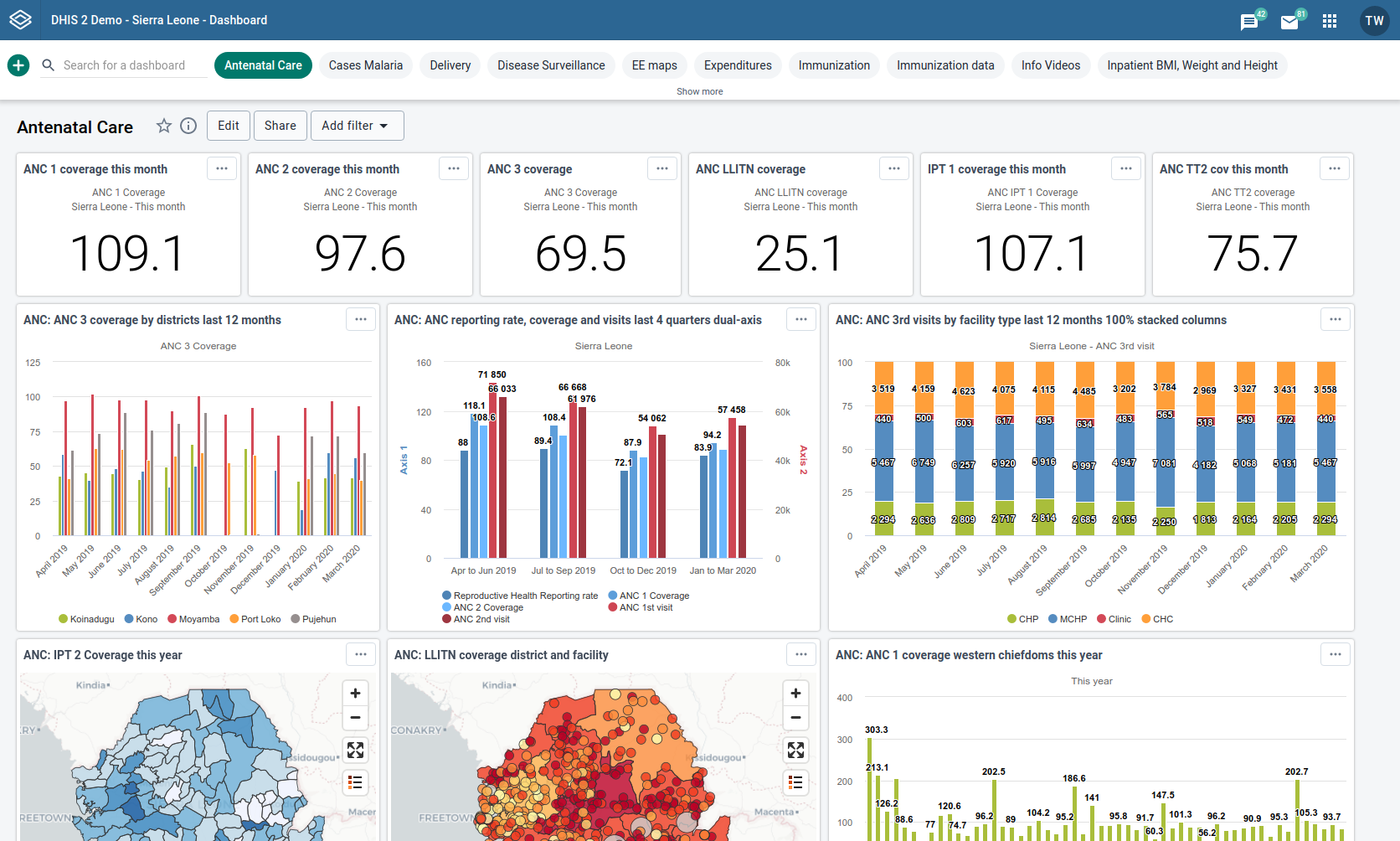
Post a Comment for "Electronic Data Capture Systems Examples"